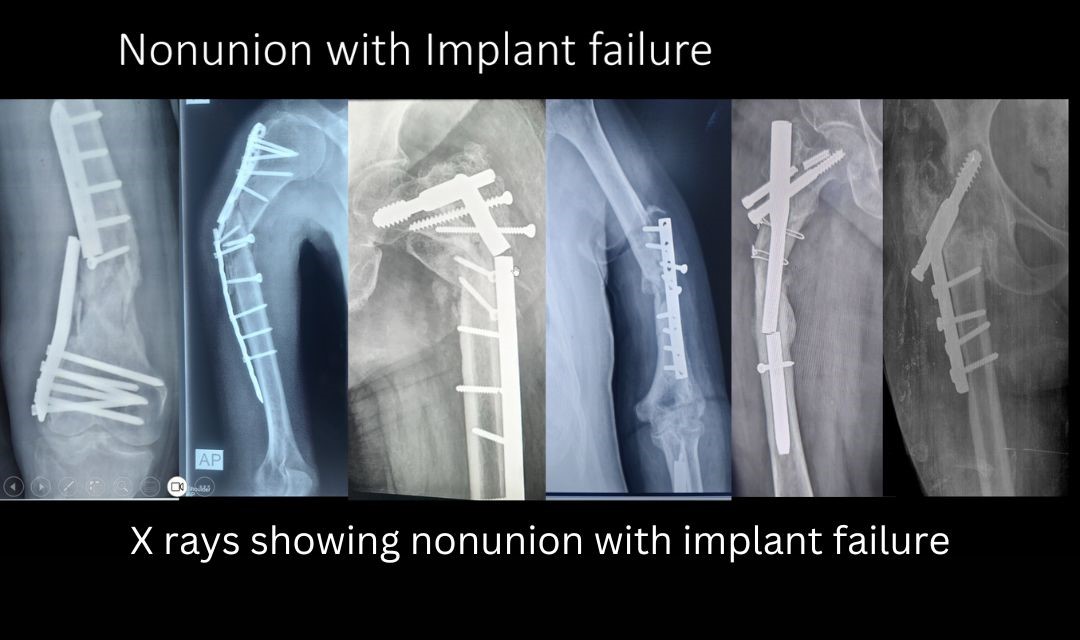
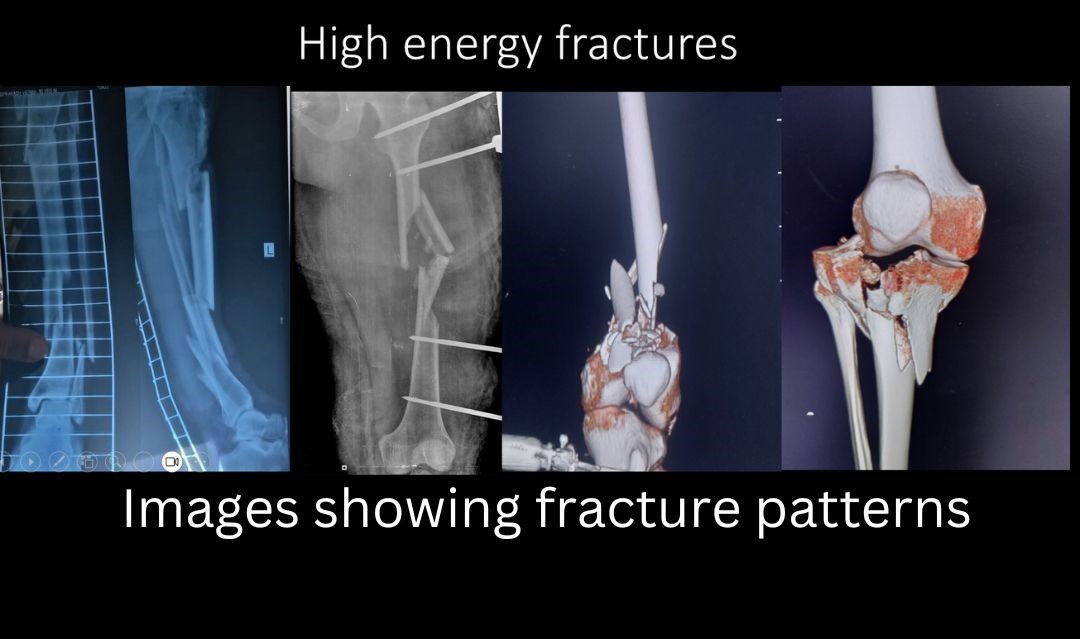
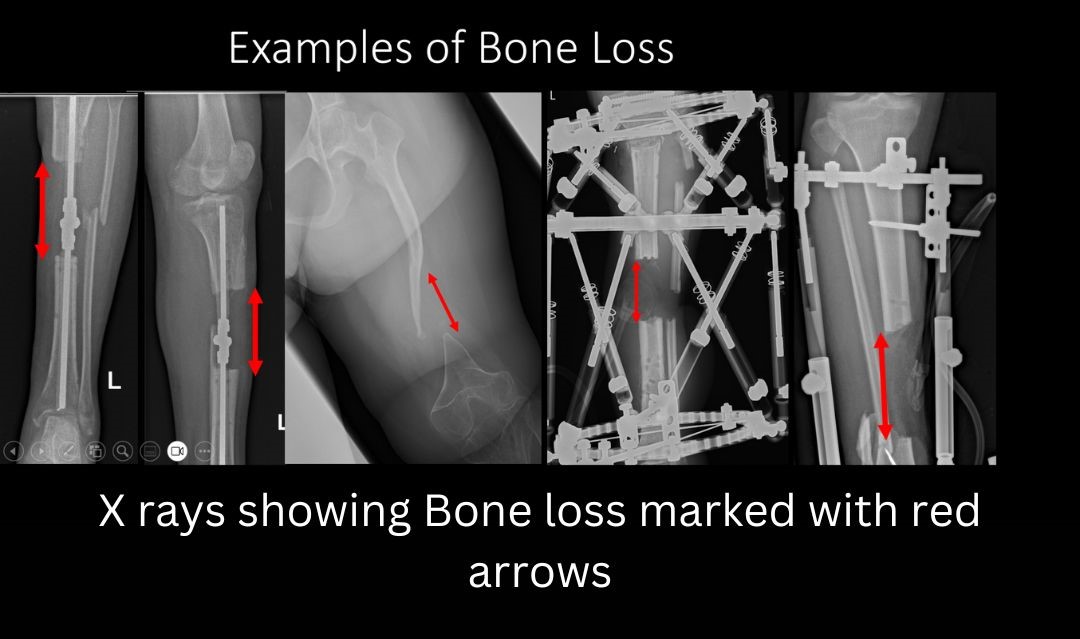
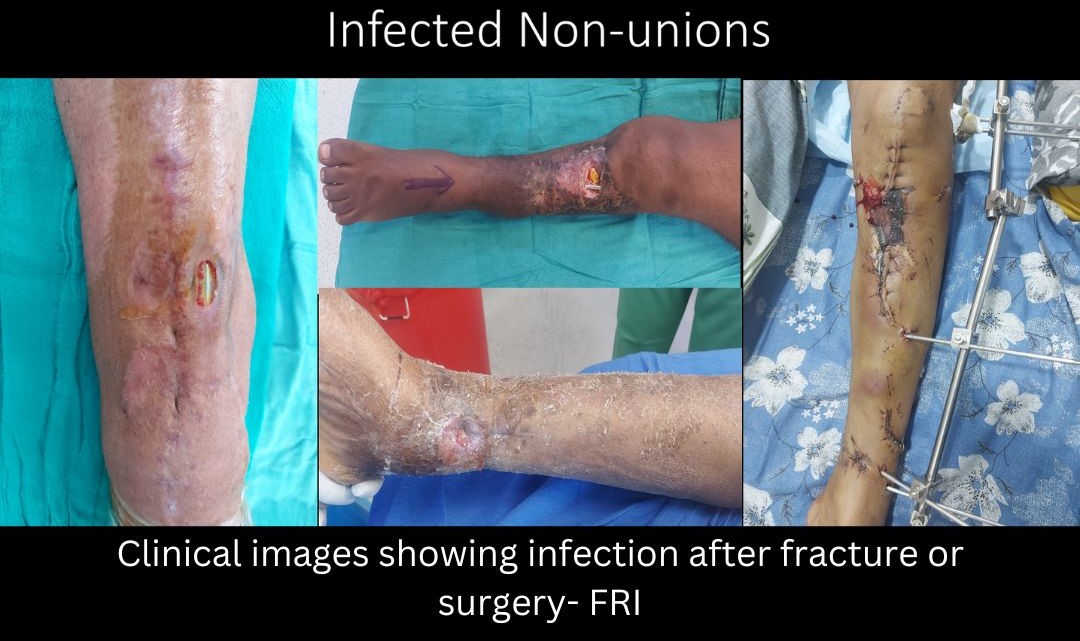
Nonunion and Management of Bone loss
Long bone fractures are one of the leading causes of morbidity and mortality around the world. Globally, in 2019, there were 178 million new fractures (an increase of 33·4% since 1990) and 455 million prevalent cases of acute or long-term symptoms of a fracture (an increase of 70·1% since 1990). These number continue to rise. It is estimated that around 2 percent of the fractures don’t unite and go into nonunion. For certain diaphyseal fractures the nonunion rate can be as high as 20 %. Nonunion have a significant impact on the functional capacity of the patient and carry an enormous financial and psychological burden. A precise definition of nonunion is difficult. According to the US FDA definition, a fracture ununited 9 months after injury or one in which there is a failure of progression towards union over the previous three months, can be classified as a nonunion. Rather than relying on a specific time frame to define a nonunion it has been proposed that a more practical definition of nonunion is a fracture that will not unite without further intervention.
Risk factors for Nonunion after fracture
Risk factors for nonunion after fracture can be divided into local and systemic factors:
- Compound fractures or fractures that breach the soft tissue and skin envelope.
- High energy injuries (pic)- like firearm, high velocity road traffic accidents
- Bone injured- Tibia or leg bone is the most common location for nonunion. Its also the most common location for Compound fractures.
- Segment of bone injured: Certain bone segments have precarious blood supply and are more prone for nonunion like distal 1/3rd of Tibia, Neck femur fracture and Scaphoid fracture.
- Bone loss: Bone loss may occur at the time of injury, or the treating surgeon may choose to remove some part of bone if its grossly contaminated and infected. Whatever the cause- bone loss is a challenging problem to manage, with a high nonunion and infection rate.
- Polytrauma and multiple fractures
- Infection: bone can become infected either at the time of injury or at the time of surgery. Late secondary hematogenous infections at the site of fracture are also known. Infection in presence of fracture is known FRI- Fracture related infection. It’s one of the most challenging orthopedic problems to manage.
Systemic or General risk factors for Nonunion
- Smoking
- Malnourishment
- Diabetes
- Age
- Obesity
- Drugs- NSAIDs and Corticosteroids, anti-convulsant medication and anti-coagulants.
- Chronic disease- CKD, CLD, Rheumatoid Arthritis
- Osteoporosis
- Vit D deficiency
- Collagen Disorders
Cause of Nonunion
Cause of nonunion can be broadly divided into two categories- biological or Mechanical. Bone needs a stable environment to heal which is provided either by surgical fixation of fractures or by Cast immobilization. Failure to provide a sound stable environment leads to nonunion. Biological problems on the other hand present as inability of the host bone to heal the fracture despite adequate stability. This can be due to necrotic or dead bone ends- resulting either from soft tissue stripping at the time of injury or infection. Some nonunion have both mechanical and biological issues.
Treatment of nonunion
It involves three steps
- Host optimization: During the pre-operative period all steps should be taken to optimize the host. These measures include- cessation of smoking, normalizing Vit D levels, controlling HbA1C levels in diabetics, treating malnourishment, cessation of drugs like NSAIDs, corticosteroids.
- Providing adequate stability to the bone- Stability to the fractured bone is provided either by internal fixation devices like intramedullary nails and or plates or by external fixation devices like circular/ ring / Linear/ hexapod fixators
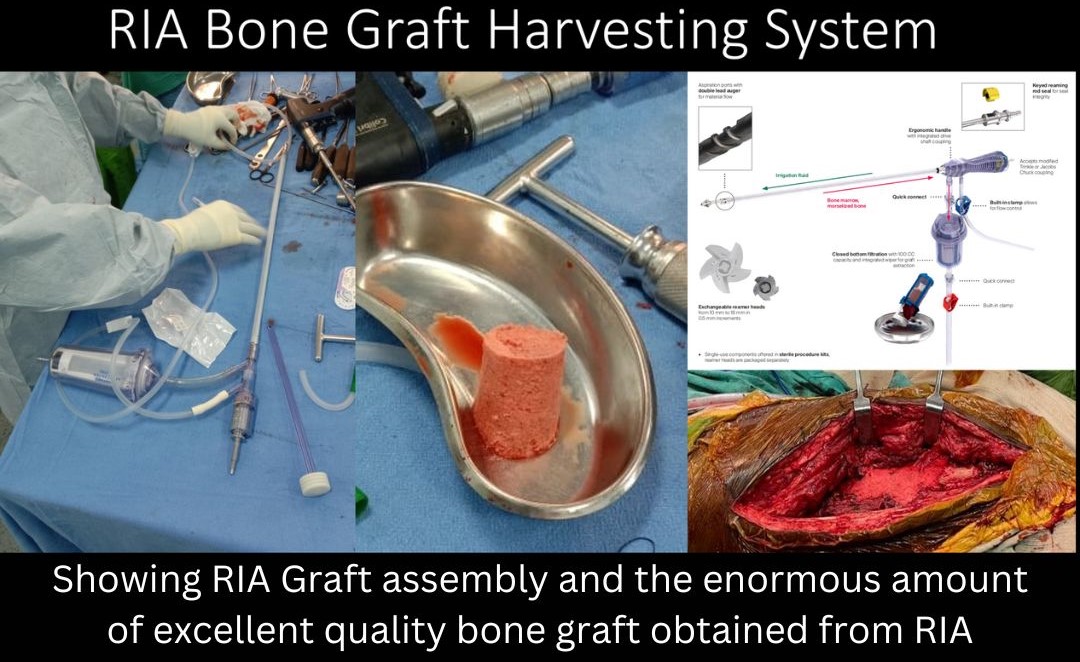
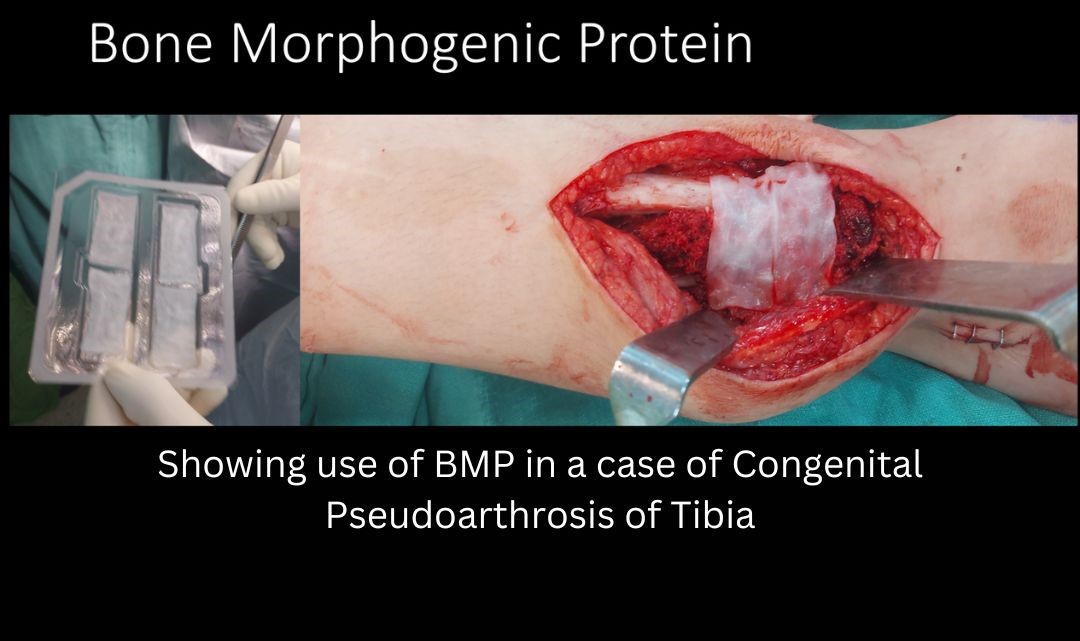
- Supplementing the local biology: The most conventional means to supplement the local biology is by adding Autogenous bone graft. Iliac crest is the usual source of bone graft. RIA (Reamer Irrigation aspirator) graft harvested from medullary canal of long bone is also an excellent source of bone graft. Advantages of RIA include minimal donor site morbidity, and a large volume of high-quality graft material. Another way to enhance the local biology is to add recombinant BMP (Bone Morphogenic Protein). BMPs are members of transforming growth factor beta super family, which possess the great osteoinductive potential. They induce osteogenesis by a sequential cascade of events resulting in fracture healing. Several clinical studies have demonstrated that recombinant human BMP-2 and rhBMP-7 improve the healing of bone defects in adults, since the Food and Drug Administration (FDA) approved their clinical use for specific indications
- Management of Bone Loss:
- Bone loss may be secondary to trauma, infection, or malignancy. Tibia and distal femur are the most sites of traumatic bone loss. A critical-sized bone defect is one which will not heal if left untreated. It’s a challenging problem facing orthopedic surgeons for centuries and has often resulted in amputation. Reconstruction of long bone defects demands a significant investment of time, finances, resources and effort, both by the patient and the surgeon. Soft tissue assessment forms a crucial part of bone loss management. Plastic surgeons form an integral part of the limb reconstruction team. Soft tissue reconstruction should be done prior to or at the same time as bony reconstruction. A healthy soft tissue bed optimizes the results of bone loss management.
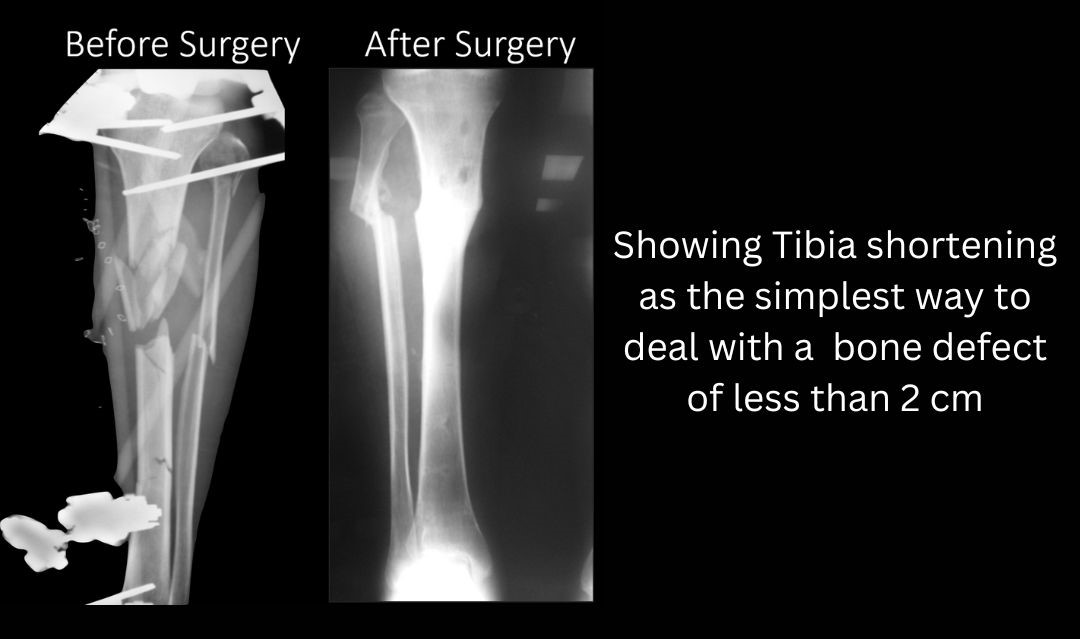
- Type 1 defect: < 2 cm in size. Shortening is the simplest way to address these bone defects and it may also lead to a healing of soft tissue without any secondary intervention. In lower limbs (Femur and Tibia) shortening up to 2cm is acceptable if no lengthening is planned. If acute shortening and gradual lengthening are planned, then tibia can be shortened by 4 cm and femur by almost 5-7 cm. The other option of dealing with Type 1 defects is either single stage cancellous bone grafting or the two stage induced membrane technique as described by Masquelet
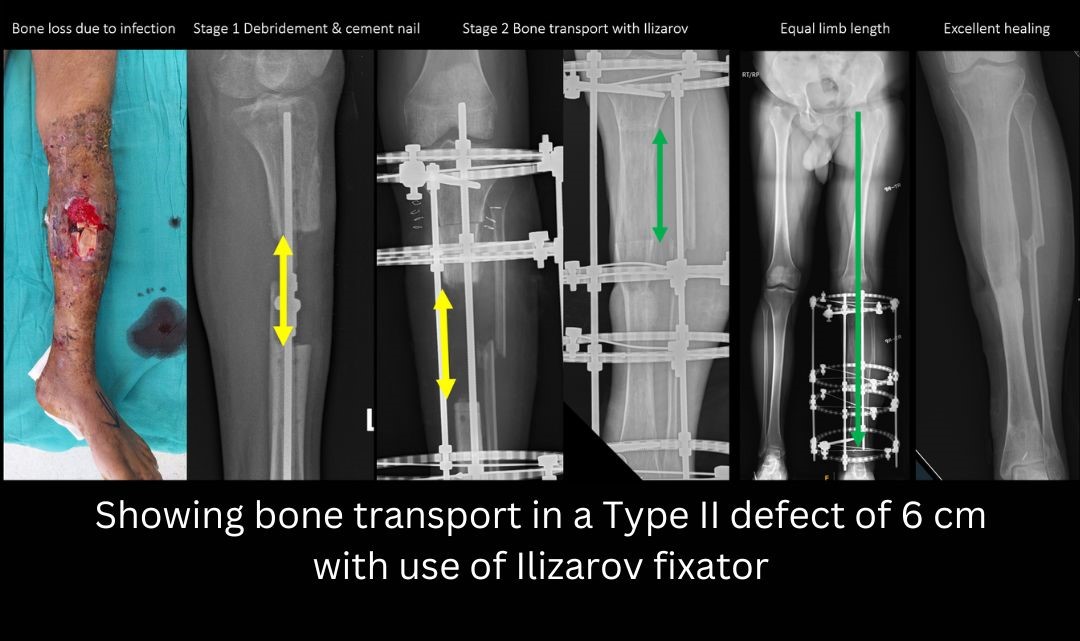
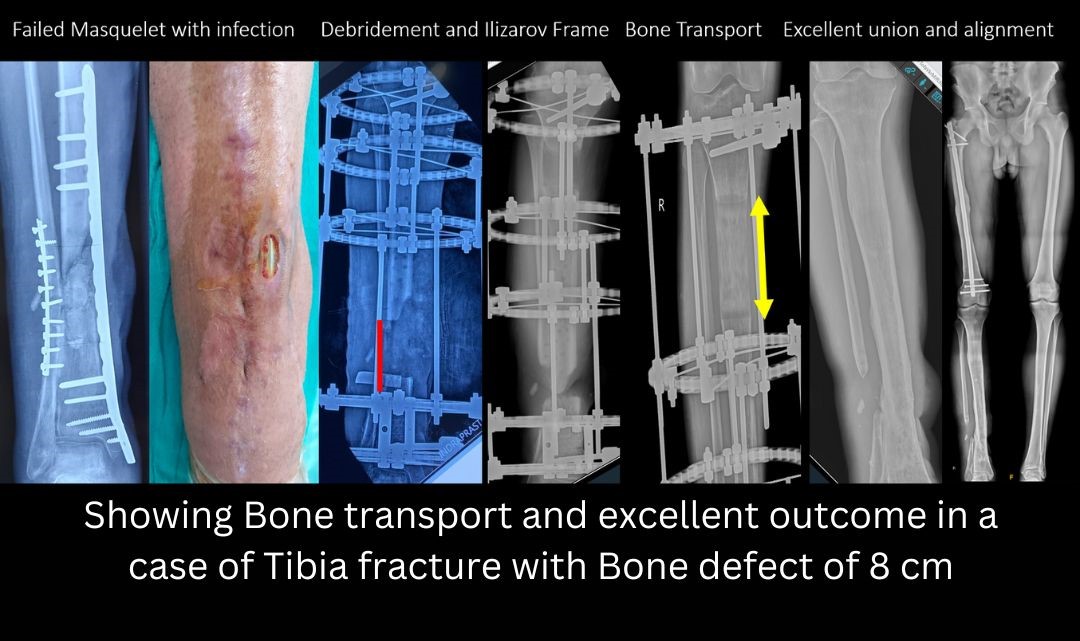
- Type 2 defect: 2-6 cms. Preferred treatment options for type 2 defects are Masquelet two stage grafting or bone transport by Ilizarov method. The Masquelet two stage procedure involves placement of a temporary cement spacer followed by a secondary grafting procedure. Placing the cement spacer gives time for the soft tissues to heal, maintains the length and space of the defect and can act as a local antibiotic carrier. The pseudomembrane which forms around the cement has immense biological potential and helps in graft incorporation.
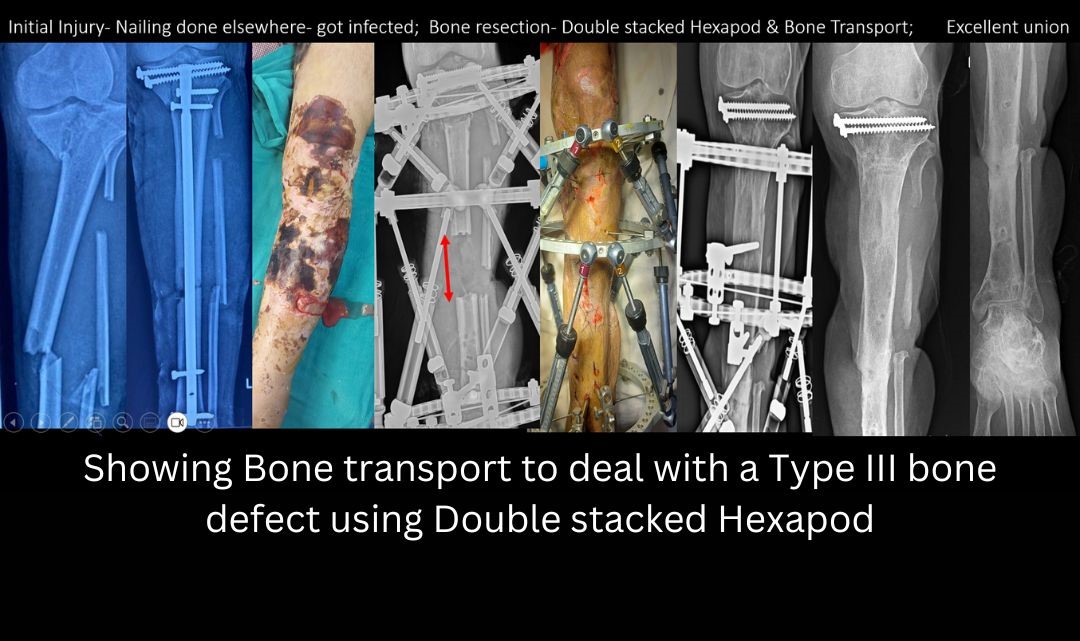
- Type III defect: 6-12 cm. Any defect > 6 cm is known as a massive defect. Treatment options include- Trifocal bone transport with Ilizarov (Two Osteotomies), Vascularized Fibula Graft, Two stage Masquelet bone grafting, and Amputation.
Based on the size of the Defect, it may be categorized as follows: